Document Type : Original article
Background: Multiple Sclerosis (MS) causes brain atrophy at the early stages of the disease which leads to progressive motor and cognitive dysfunction. Brain atrophy can be diagnosed indirectly by measuring the Third Ventricle Diameter (TVD) using Trans Cranial Sonography (TCS). The purpose of the current study was evaluation of TVD in MS patients using TCS to examine its possible correlation with cognitive dysfunction and Expanded Disability Status Scale (EDSS).
Methods: Seventy-four patients with a definite diagnosis of MS were enrolled in this study. Transverse diameter of the third ventricle was measured using TCS. All patients were assessed by neurological examination and the level of disability was measured via EDSS. The cognitive performance was assessed by the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Pearson’s correlation was performed to evaluate possible correlations and p-value<0.05 was considered statistically significant.
Results: From the total 74 individuals, 58.1% were diagnosed with Relapse-Remitting MS (RRMS) (n=43) and 43.9% with Secondary-Progressive MS (SPMS) (n=31). The mean EDSS score was 1.81±1.38 (Range of 0-10). The mean TVD was 5.61±1.82 cm which had no statistical correlation with the EDSS score, but it was significantly wider in group with EDSS score>3 when compared to the group with EDSS score≤3 (p-value=0.0001). The mean BICAMS score was 0.65±0.57 and there was no statistical correlation between BICAMS score and TVD.
Conclusion: Measuring the diameter of the third ventricle using TCS appears to be an appropriate method to diagnose brain atrophy and is associated with cognitive dysfunction in the process of MS. Our data emphasized no association between the diameter of the third ventricle and cognitive dysfunction.
Keywords: Cognitive dysfunction, Humans, Motor disorders, Multiple sclerosis, Neuroimaging, Third ventricle
Introduction
Multiple Sclerosis (MS) is known as an immune-mediated disease, characterized by multifocal inflammation of the central nervous system, extensive axonal transection, demyelination, and neurodegeneration, whichleads to loss of brain volume (1,2). Brain atrophy in MS patients appears at the early stages of the disease and has a faster progression than normal aging processes (3-5).
Studies have shown that brain atrophy affects evolution of motor abilities and induces cognitive disabilities in MS (6-9). Therefore, some studies have been conductedon the evaluation of cerebral atrophy viaserial Magnetic Resonance Imaging (MRI) to predict physical and cognitive dysfunction in patients suffering from MS as well as the effectiveness of the treatment (7,8,10-12). Trans Cranial Sonography (TCS) of brain parenchyma is another available method of neuroimaging,by which several quick assessments can be done and deep brain structure at high resolution can be displayed (13). Indirect evaluation of brain atrophy can be done by measuring the diameter of the third ventricle using TCS (14).
Several researchers have suggested that the diameter of the third ventricle can be used as an alternative for the prediction of the disability progression and brain atrophy over the disease course (14-16). It has previously been shown that clinical parameters of disability, such as the Expanded Disability Status Scale (EDSS), correlate with the diameter of the third ventricle (14,15).
In the present study, an attempt was made to evaluate the diameter of the third ventricle in MS patients using TCS and find whether there is a correlation between clinical parameters of cognitive and motor disability, such as the EDSS and the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS).
Materials and Methods
Patients and study design
In this cross-sectional analytic investigation, patients with a definite diagnosis of MS according to the Mc Donald criteria (17) were enrolled by simple random sampling from the Multiple Sclerosis outpatient clinic of Firoozgar Hospital between 2018-21. They were excluded from the study if they had:1) any history of attack in the last 3 months, 2) mental or severe physical disability, 3) illiteracy or a condition that limits the ability to read (e.g.blindness or severe visual impairment) Seventy-four individuals (including 15 men, 59 women, in the range of 17 to 60 years, and a mean age of 35.95±10.84 years) were enrolled in the final analysis. This study was approved by the Ethics Committee of Iran University of Medical Sciences (Ethics code: IR.IUMS.FMD.REC.1396.9511158006). All the patients who met the criteria were informed about the study and ethical consent was obtained.
Data collection and patient examination
The collected data included demographic characteristics, duration of the disease, disease course [Relapsing-Remitting (RR), Secondary-Progressive (SP), Primary-Progressive (PP), and Progressive-Relapsing (PR) MS] and their current treatment protocol (since the last 2 years) was recorded in prepared checklists. All the patients were assessed by neurological examination and via EDSS to check the disease severity. Furthermore, cognitive domains were assessed by BICAMS (18).
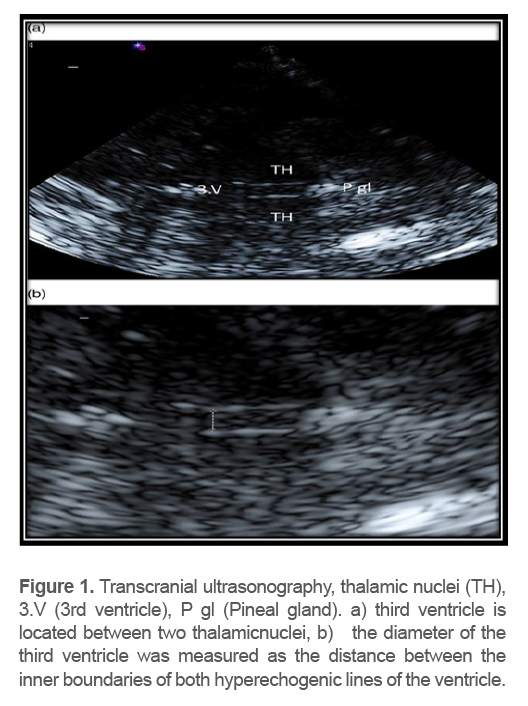
TCS was performed by an experience dneur ologist using a 2 MHz phased array transducer. Next, a transtemporal window inthe axial imaging plane was used. The width of the third ventricle was assessed at the thalamus level and measured as the distance of the leading edges of the brain-ventricle interfaces in axial image planes (Figure 1).
Statistical analysis
Data analysis was performed using SPSS v 22.0 (IBM,USA). All the data were reported as mean±standard deviation (SD). Anindependent samplest-test was performed to compare variables. To test the normal distribution of variables, the Kolmogorov–Smirnov test was carried out and Spearman’s correlation was performed to evaluate the possible correlations. A p-value <0.05 was considered statistically significant.
Results
In general, 58.1% of the total 74 individuals were diagnosed with RRMS (n=43) and 43.9% with SPMS (n=31). The patient’s characteristics, the mean duration of the disease, and the mean EDSS score (Range of 0-10) are summarized in table 1. Our data showed that EDSS score has a significant correlation with duration of the disease (p=00r=0.52) and age (p-value=00,r=0.46). But the mean EDSS score did not significantly vary between men and women (p-value>0.05). Moreover, no association was found between EDSS score and Third Ventricle Diameter (TVD) (p-value >0.05).
Table1. Patients’course of disease, EDSS score, and disease duration
|
Disease duration Mean± SD |
EDSS Mean± SD |
SPMS n(%) |
RRMS n(%) |
|
|
3.19±2.382 |
1.73±1.03 |
6 (8.1%) |
9 (12.1%) |
Males |
|
7.38±7.26 |
1.83±1.46 |
25(33.7%) |
34 (45.9% |
Females |
|
6.53± 6.77 |
1.81±1.38 |
31(43.9%) |
(58.1%) 43 |
Total |
Mean TVD was 5.61±1.82 cm. Spearman’s correlation showed a statistical correlation between TVD with the duration of the disease (p-value=0.003, r=0.34), as well as patient’s age (p-value=0.004, r=0.34), but not with EDSS score (p-value>0.05). However, it was significantly wider in the group with EDSS score>3 when compared to the group with EDSS score≤3 (p-value=0.0001). The mean diameter of the third ventricle was not different in the disease courses (p-value>0.05).
Mean BICAMS score was 0.65±0.57, which was not statistically different in RRMS and PSMS patients (BICAMS=0.65±0.6 and 0.66±0.54, respectively, p-value=0.25). Furthermore, there were no differences in BICAMS scores when compared in groups with age>40 and ≤40 (p-value=0.61), and no differences were found in BICAMS scores when compared in groups with EDSS score<3 and ≥3 (p-value=0.92, data not shown). BICAMS score had no correlations with EDSS score, age, and the duration of the disease (All p-values>0.05). However, BICAMS score in the subjects with a duration of thedisease under 1 year was statistically different from those with duration over 1year (p-value=0.003). There were no statistical correlations between BICAMS score and TVD (data not shown, p-value>0.05).
Most of the patients in our study used Cinnovex (interferon-beta 1a) and Rituximab in the last two years. In a one-way analysis with 95% CI, there were no significant differences between the types of the drugs and ventricular diameter (p-value=0.22). There were also no statistical differences of the mean BICAMS scores in different drugs used currently and the drugs used in the last two years (p-value=0.47).
Discussion
In the current study, the purpose was to find whether TCS measure ment of the third ventricle is correlated with cognitive or motor dysfunction. Researchers have suggested that diameter of the third ventricle is a valuable marker for early diagnosis of brain atrophy and leading disabilities which can indicate progression of disease in patients with MS (15,19). Berg et al indicated a significant correlation between TVD and disability measures by EDSS (15). This was then proved by other studies (14,19,20). Supporting these data, our results showed that the third ventricle was significantly wider in the group with EDSS score>3 as compared to group with EDSS score≤3 (p-value=0.0001). However, correlation tests did not show any associations with TVD and EDSS score (p-value=0.2). This discrepancy may be due to thelow overall EDSS score of our study population (Mean ±SD=1.81±1.38).
Previous studies demonstrated thatthe patient’s medication did not have any associations with the ventricular diameter (20), which was confirmed by our results (p-value>0.05).
The BICAMS includes tests of mental processing speed and memory. However, it is considered to be a cognitive assessment for MS patients (18). This study did not demonstrate significant correlation between BICAMS score and ventricular diameter and EDSS score. This might be due to lack of abnormal BICAMS scores in our patients, which was due to our small sample size and low EDSS score (the mean EDSS was 1.81); in fact, a less severe disability in motor function was observed in this study. There has been a relationship between cognitive decline and motor disability in previous studies (6,21).
Berg et al argued that TCS and MRI measuring the third ventricle correlated significantly with most of the neuropsychological tests and they found no meaningful correlations between the diameter of the ventricles and depression scales (15). Even though neurodegenerati on begins in SPMS patients, which may lead to brain atrophy, no relationship was found between the disease courses and TVD. However, it was found that TVD had a direct relationship with motor dysfunction (measured with EDSS score>3) and disease duration.
The role of TCS in the measurement of TVD and diagnosis of neurodegenerative disease has been demonstrated by previous research (22). Since previous studies have demonstrated a significant correlation between MS and results of TVD, it is safe to say that TCS, as a reliable measuring tool, can be used to predict brain atrophy ina single point study. Furthermore, it is not useful to monitor disease progression particularly at the very early stages of the disease (19).
Conclusion
Measuring the diameter of the third ventricle using transcranial sonography appears to be an appropriate method to find brain atrophy and is associated with disease duration and advanced disability in the process of MS neurodegeneration. Further studies with adequate sample and less dispersion of patients should be conducted to clarify the relationship between the rate of brain atrophy and cognitive decline in MS.
Funding
This study was funded by a research grant from Iran University of Medical Sciences, Tehran, Iran.
Acknowledgements
We would like to thank Dr.Babak Zamani for his kind support and guidance.
Conflict of Interest
All authors certify that they have affiliation with or any involvement in any organization or entitywith any financial interest or non-financial interest in the subject and material discussed in this manuscript.